A Student Balances the Following Redox Reaction Using Half-reactions.
Balance redox reactions using the half-reaction method. What is necessary is that the half-reactions be separately balanced both by mass and charge before the final addition.

Balancing Redox Equations Half Reactions Basic Solutions Equations Redox Reactions Solutions
Balancing Redox Equations Using Half-Reactions An overall redox equation can be obtained by.
. Balance the given redox reaction. Balance the two half-reactions separately. In using the half-reaction method assume that the reaction occurs in an aqueous acid solution Fe2O3 CO Fe CO2 acid solution.
As noted previously charge balance occurs after mass balance and only uses electrons. Basic solutions of potassium permanganate and sodium sulfite are added. Balance the following oxidation-reduction redox reactions using the half reactions method.
Balance the following redox reaction in basic solution. 3 on a question A student balances the following redox reaction using half-reactions. MnO 4- I - --- Mn 2 I 2 H 2 O Oxidation half is.
All three reactions are acidic and the ionic species in the reaction are aqueous. Calculate AS rxn for the following reaction. Lanay369 lanay369 09242019 Chemistry High School answered A student balances the following redox reaction using half-reactions.
Split the skeletal equation into two half-reactions- oxidation half-reaction and reduction half-reaction. 2 HNO3 aq. How many electrons will be lost in all.
Balance the following redox reaction in basic solution. The two half-reactions are then multiplied by suitable integers so that the total number of electrons gained in the reduction half-reaction is equal to the total number of electrons lost in the oxidation half-reaction. Upper A l plus upper M n superscript 2 plus right arrow upper A l superscript 3 plus plus upper M n.
CuS s NO3- aq Cu² aq S s NO g Enter your. Balance the following redox reaction using the method of half-reactions NO aq Sn 2 - NO So ay acidic solution 6. A student is asked to balance an equation by using the half-reaction method.
Ir3 aq Hg 1Ir s Hg2² aq a To show your method write the balanced half reactions below. H2 O22- - H2O. Balance the redox reaction using half reaction method MnO 4-I-MnO 2 IO 3 - in basic medium.
Use the smallest integer coefficients possible and show electrons as e. Balance the following redox equation using either the oxidation-number-change method or the half-reaction method. Which is an important step in the alternate method for balancing equations in redox reactions.
I- 3H 2 O. 3 Write the balanced oxidation half reaction. S2O32- IO3- Ã SO42- I-2.
Though the oxidation half-reaction was written first in Figure 2 this is not necessary. How many electrons will be lost in all. Balance the following redox reaction in basic solution using the half reactions method.
If a box is not needed leave it blank. All three reactions are acidic and the ionic species in the reaction are aqueousa. 1 Assign oxidation number to all elements.
MnO 4 SO 3 2- H 2 O MnO 2 SO 4 2- OH 2 MnO 4 3 SO 3 2- H 2 O 2 MnO 2 3 SO 4 2- 2 OH b. Get the answers you need now. Breaking into separate reduction and oxidation half-reactions 2.
2 Write the balanced reduction half reaction. On a piece of paper balance and then add the following half-reactions to yield an. Chemistry questions and answers.
Consider the following reaction. Click hereto get an answer to your question Balance the following redox reactions by the ion - electron method in basis medium MnO-4aq I-aq MnO2s I2s. NO3- Cus à NOg Cu2 b.
Correct answer to the question A student balances the following redox reaction using half-reactions. Balancing each half 3. VO 2 MnO 4----- VOH 4 Mn 2.
A solution of Iron II nitrate is added to a basic solution of hydrogen peroxide. Balance the following redox reactions using the half-reaction method. The Sº for each species is shown below the reaction 4 NH3 g 502 - 4 NO g 6H2O g SJmol K 1928 2052 2108 1888 7.
Share with your friends. Upper A l plus upper M n superscript 2 plus right arrow upper A l superscript 3 plus plus upper M n. S2O32- IO3- àSO42- I-2.
Balance the following oxidation-reduction redox reactions using the half reactions method. Balance the redox reaction below using the half-reaction method. Balance the following Redox reactions using the half reaction method.
Zn s NO3- aq Zn2 aq NH3 aq Enter your chemical notation here Balance the following redox reaction in acidic solution using the half reactions method. A student balances the following redox reaction using half-reactions. Balance the following redox reaction.
ClO 4- I 2 Cl- IO 3-Balanced. Label it using the dipole. NO3- Cu s àNO g Cu2b.
He determines the two half reactions as shown below. You must show all your work especially the total number of electrons transferred in the reaction. Considering the equation above we have 2 hydrogen H with the total charge 1 Refer the charges of the elements in the above table and 2 oxygen O with the total charge -2 on the LHS and 2 hydrogen H with total charge 2 and only 1 oxygen O with the total charge -2 on the RHS.
Ps SO42- Ã PO43- SO32. The balanced reaction is given as. Consider the redox reaction below.
Adding the two half reactions together once the number of e- lost in oxidation are balanced by the e- gained by reduction ex. 4 Balance the ionic equation.

Balancing Half Redox Equations Equations Chemistry Positivity

Redox Reactions Classwork Homework Redox Reactions Covalent Bonding Chemical Equation

Tang 02 Balancing Redox Reactions 2 Redox Reactions Chemistry Lessons Teaching Chemistry
How To Balance Redox Equations In Basic Solution Schooltube Safe Video Sharing And Management For K12
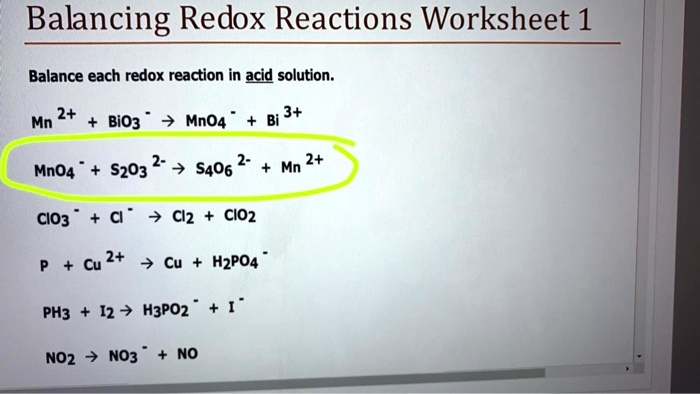
Solved Balancing Redox Reactions Worksheet 1 Balance Each Redox Reaction In Acid Solution 2 Mn 3 Bio3 Mno4 Mno4 S203 2 7 S406 2 Mn 2 Clo3 Cl 7 Clz Cio2

Tang 02 Balancing Redox Reactions 2 Redox Reactions Reactions Organic Reactions

Balancing Redox Reactions Using The Ion Electron Method Youtube

Pin By Saitech Informatics On Balancing Of Chemical Reaction Chemistry Worksheets Equations Redox Reactions

Pin On Science With Tyler Dewitt
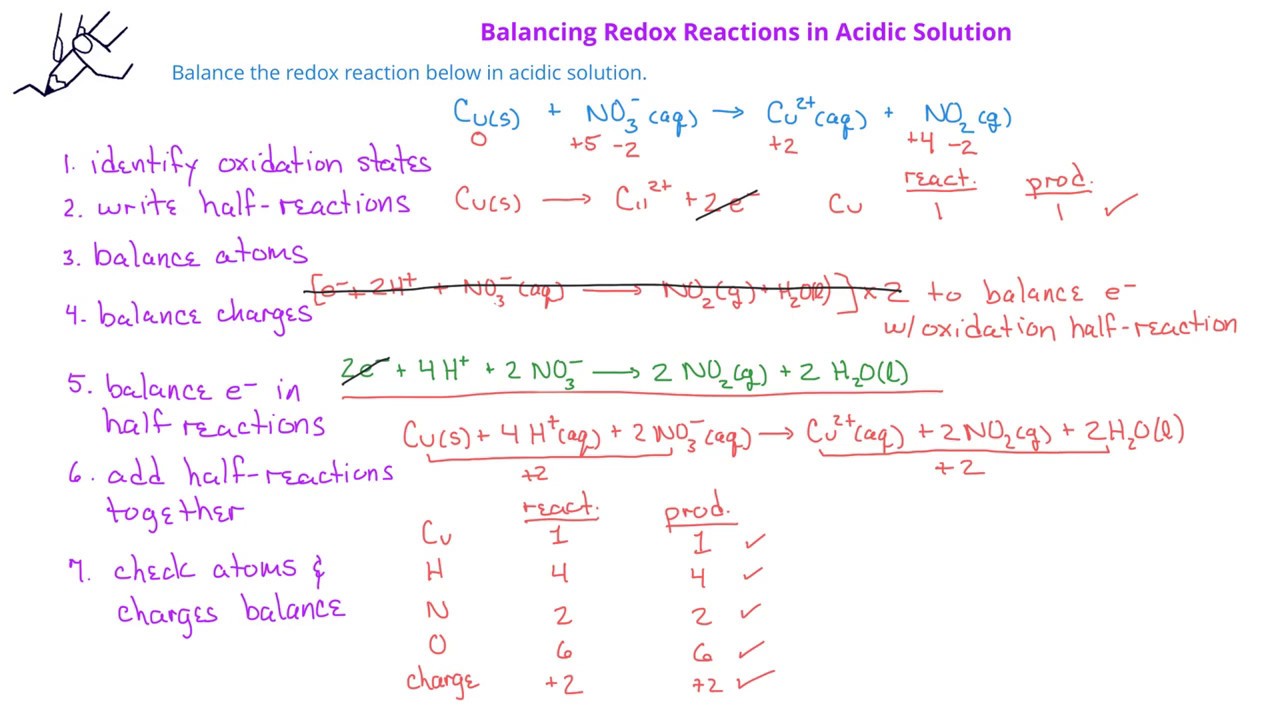
Redoox Reactions 03 Balancing Redox Reactions In Acidic Solution Youtube

Balance A Redox Reaction Basic Solution Redox Reactions Ap Chemistry Chemistry
Lesson 2 Balancing Redox Equations Grade12uchemistry
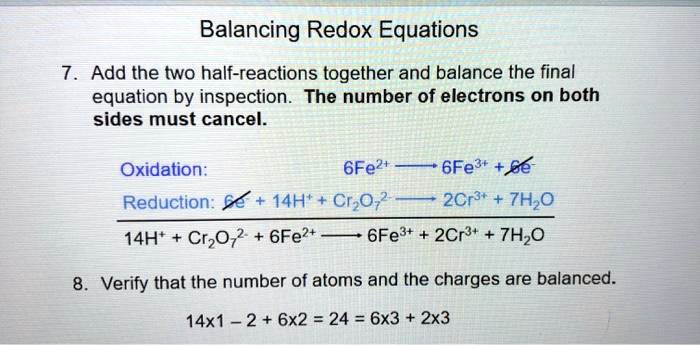
Solved Balancing Redox Equations Add The Two Half Reactions Together And Balance The Final Equation By Inspection The Number Of Electrons On Both Sides Must Cancel Oxidation 6fe2t 6fe3t Be Reduction 14h
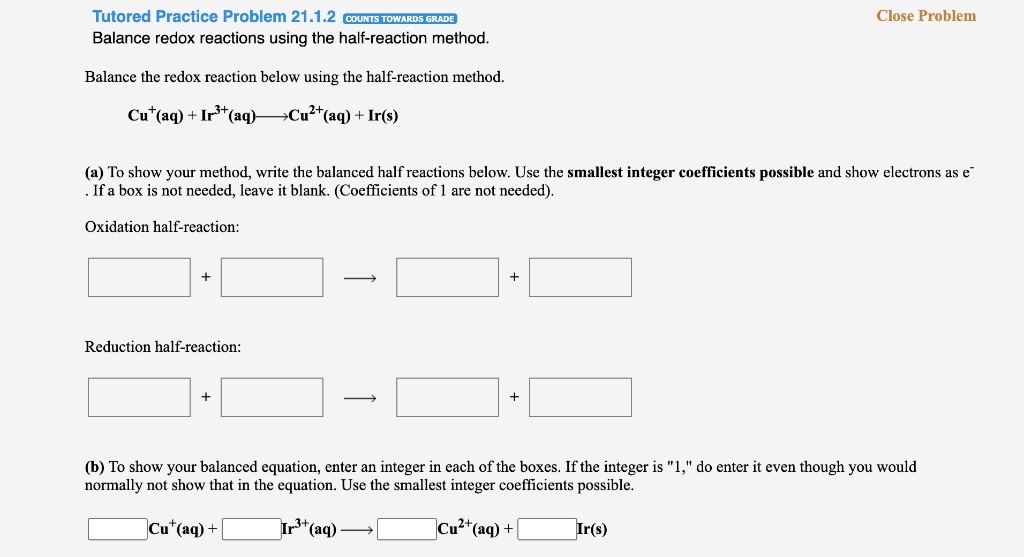
Solved Tutored Practice Problem 21 1 2 Counis Towards Gradb Balance Redox Reactions Using The Half Reaction Method Close Problem Balance The Redox Reaction Below Using The Half Reaction Method Cu At Aq Irst Aq Cult Aq Ir S A To

Balancing Redox Reactions 2 Vcc Library

Click To Download Redox Rules Posters For Vce Chemistry Teaching Chemistry High School Science Ap Chemistry



Comments
Post a Comment